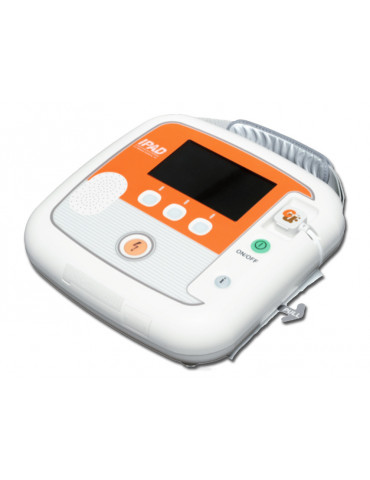
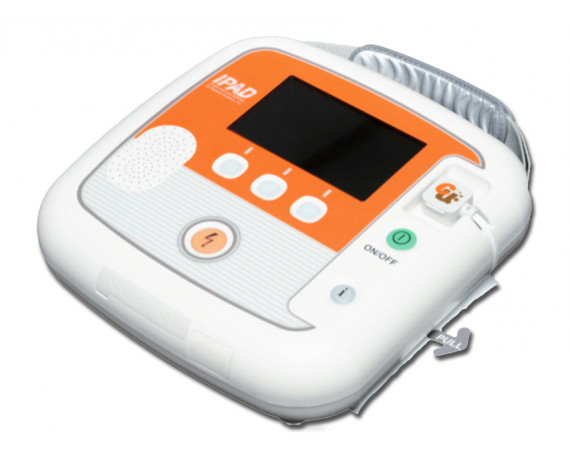
Semi-automatic iPAD CU-SP2 CU Medical defibrillator with monitor
Semi-automatic defibrillator with monitor model iPAD CU-SP2 manufactured by CU Medical available in 25 languages.
 Are you in UK or outside Euro Zone?
Are you in UK or outside Euro Zone?
You will not pay Italian 22% VAT
 Are you a Doctor or a Professional?
Are you a Doctor or a Professional?
Sign in here to obtain reserved price list
 Fast shipping throughout Europe
Fast shipping throughout Europe
We use only premium couriers
Semi-automatic defibrillator with monitor model iPAD CU-SP2 produced by CU Medical available in 25 languages, to be specified when ordering .
Semi-automatic external defibrillator applicable to patients with sudden cardiac arrest (SCA). CU-SP2 is a battery-operated, easy-to-use, compact, lightweight and portable defibrillator. The AED automatically reads the patient's electrocardiogram (ECG) and determines whether a cardiac arrest (SCA) requiring defibrillation has occurred, making it easy to use by both emergency medical technicians, medical professionals and non-specialized users.
- Automatic volume adjustment
It automatically adjusts its volume in proportion to the noise emitted in the surrounding environment.
- Pre-connected plates for a defibrillator always ready for use
It is equipped with pre-connected plates housed in a special compartment located under the defibrillator. This ensures that the defibrillator is ready for use in the shortest possible time. It is also able to perform a precise check on the life status of the plates themselves. The front of the defibrillator, in fact, houses a light indicator that changes color both 3 months before the plates expire, and when the plates have expired.
- CPR detection
CPR is vital to ensure the patient has the best chance of survival. The iPAD CU-SP2 detects whether the rescuer is performing CPR or not and prompts the rescuer to perform CPR or continue CPR, respectively.
- Easy communication with CU-EX1 software
Allows you to view and analyze information such as power-on time, patient heart rate, and shocks applied. Stores 3 events in internal memory (up to 17 hours per event).
- Wireless ECG transmission device (CU-EM1) - optional
In monitoring mode, iPAD CU-SP2 uses Bluetooth to receive ECG data from CU-EM1 and displays it on the LCD screen.
- Printer - optional
iPAD CU-SP2 supports connection with external Bluetooth printer. Software voice: US, GB, FR, IT, ES, PT, DE, PL, CZ , KR
Online Manual: US, GB, FR, IT, ES, PT, DE, CZ, KR (For languages listed in "Software Voice" only, the manual is in English. Paper manual for GB, IT only)
br>
Technical features
- Mode: Semi-automatic
- Waveform: Biphasic Truncated Exponential
- Energy: AED mode: 150/200J fixed or 150-150-200J increasing energy
- Charging time: <10 seconds
- Charging time after end of CPR: At least 6 seconds
- Waterproof: Protection against water jets IPX5 according to IEC60529 (IP55)
- Dust protection IP5X according to IEC60529
- Dimensions - Weight: 260x256x69.5 mm - 2.4 kg
User Interface
- User Support: Detailed voice and text messages
- CPR Guide: Voice prompts show how to perform CPR on adults and children
- Controls: On/off button, “i” button, discharge button, programmable buttons
- Indicators: Graphic LCD display (status, user guide, ECG, heart rate)
- Sensors: Plate expiration date, plate connection status
- CPR monitoring, ECG monitoring, automatic volume adjustment
Data recording and transmission
- IrDA Port: Wireless data transmission to PC, SD Card
- Internal memory (Nand-flash): ECG, events
- Memory capacity: Multi recordings 3 events / Max 17 hours per event
- Bluetooth: Communication with printer or ECG CU-EM1
- Data review PC program: CU-EX1
Patient analysis system
- Patient Analysis: Shockable Rhythms - Ventricular Fibrillation, Tachycardia
- Sensitivity/Specificity Conforms to AAMI DF80 guideline
Drums
- Standard Type: DC 12 Volt 4.2 Ah, LiMnO2
- Capacity: Minimum 200 shocks (150J)
- Rechargeable (optional) Type: DC 11.1 Volt 1.9 Ah, Lithium-ion, rechargeable
- Capacity: Minimum 60 discharges or 3 hours of operation
Standard equipment
- Adult plates
- Disposable lithium battery
- Transport bag
- Paper manual (GB, IT)
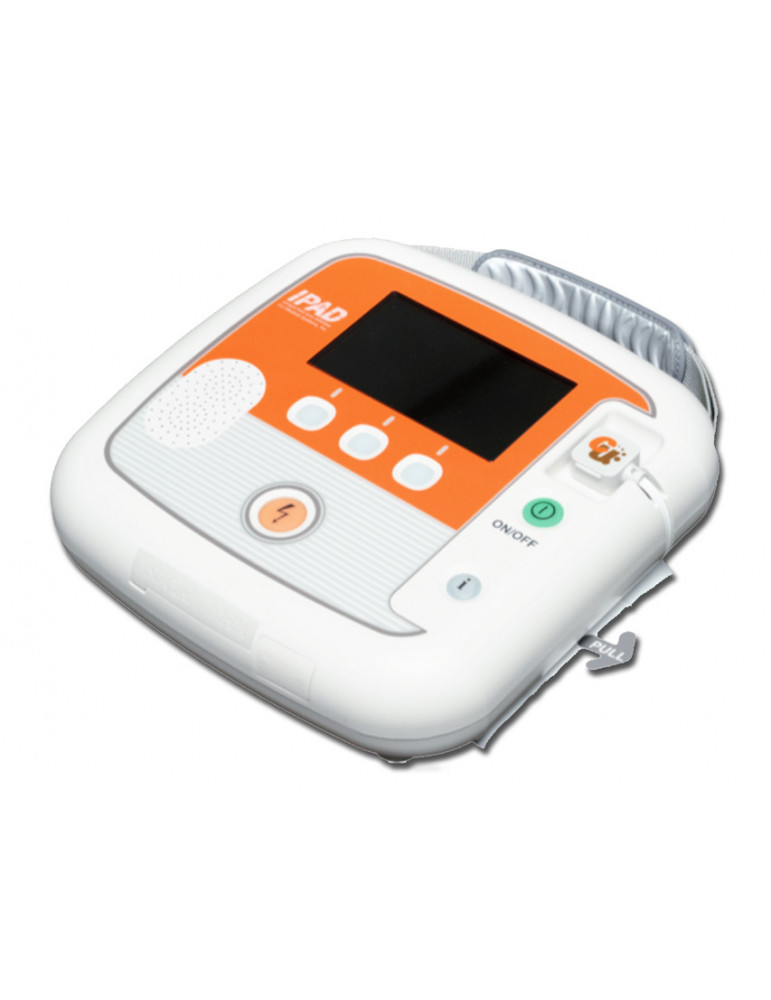
Semi-automatic defibrillator with monitor model iPAD CU-SP2 manufactured by CU Medical available in 25 languages.